# Load
library(tidyverse)
library(tidybayes)
library(rstan)
library(loo)
library(patchwork)
library(posterior)
# Drop grid lines
theme_set(
theme_gray() +
theme(panel.grid = element_blank())
)11 God Spiked the Integers
Load the packages.
11.1 Binomial regression
11.1.2 Relative shark and absolute deer
Based on the full model, m11.4, here’s how you might compute the posterior mean and 89% intervals for the proportional odds of switching from treatment == 2 to treatment == 4.
as_draws_df(m11.4) |>
mutate(proportional_odds = exp(`b[4]` - `b[2]`)) |>
mean_qi(proportional_odds, .width = 0.89)# A tibble: 1 × 6
proportional_odds .lower .upper .width .point .interval
<dbl> <dbl> <dbl> <dbl> <chr> <chr>
1 0.929 0.574 1.39 0.89 mean qi A limitation of relative measures measures like proportional odds is they ignore what you might think of as the reference or the baseline.
11.1.2.1 Overthinking: Proportional odds and relative risk
11.1.3 Aggregated binomial: Chimpanzees again, condensed
With the tidyverse, we can use group_by() and summarise() to achieve what McElreath did with aggregate().
d_aggregated <- d |>
group_by(treatment, actor, side, cond) |>
summarise(left_pulls = sum(pulled_left)) |>
ungroup()
# What?
d_aggregated |>
head(n = 10)# A tibble: 10 × 5
treatment actor side cond left_pulls
<fct> <int> <dbl> <dbl> <int>
1 1 1 1 1 6
2 1 2 1 1 18
3 1 3 1 1 5
4 1 4 1 1 6
5 1 5 1 1 6
6 1 6 1 1 14
7 1 7 1 1 14
8 2 1 2 1 9
9 2 2 2 1 18
10 2 3 2 1 11Update the stan_data for the new d_aggregated format.
stan_data <- d_aggregated |>
compose_data(n_actor = n_distinct(d_aggregated$actor))
# What?
str(stan_data)List of 8
$ treatment : num [1:28(1d)] 1 1 1 1 1 1 1 2 2 2 ...
$ n_treatment: int 4
$ actor : int [1:28(1d)] 1 2 3 4 5 6 7 1 2 3 ...
$ side : num [1:28(1d)] 1 1 1 1 1 1 1 2 2 2 ...
$ cond : num [1:28(1d)] 1 1 1 1 1 1 1 1 1 1 ...
$ left_pulls : int [1:28(1d)] 6 18 5 6 6 14 14 9 18 11 ...
$ n : int 28
$ n_actor : int 7Define model_code_11.6 and fit the model. As an alternative, try fitting the model with the binomial_logit() and binomial_logit_lpmf() functions like we did for m11.5, above. Which method has better HMC chain diagnostics?
model_code_11.6 <- '
data {
int<lower=1> n;
int<lower=1> n_actor;
int<lower=1> n_treatment;
array[n] int treatment;
array[n] int actor;
array[n] int<lower=0, upper=18> left_pulls;
}
parameters {
vector[n_actor] a;
vector[n_treatment] b;
}
model {
left_pulls ~ binomial(18, inv_logit(a[actor] + b[treatment]));
// left_pulls ~ binomial_logit(18, a[actor] + b[treatment]);
a ~ normal(0, 1.5);
b ~ normal(0, 0.5);
}
generated quantities {
vector[n] eta;
vector[n] log_lik;
eta = a[actor] + b[treatment];
for (i in 1:n) log_lik[i] = binomial_lpmf(left_pulls[i] | 18, inv_logit(eta[i]));
// for (i in 1:n) log_lik[i] = binomial_logit_lpmf(left_pulls[i] | 18, eta[i]);
}
'
m11.6 <- stan(
model_code = model_code_11.6,
data = stan_data,
cores = 4, seed = 11)Rather than the typical print() summary, here we’ll compare the a and b parameters in this aggregated binomial model m11.6 with its disaggregated version m11.4 in a coefficient plot.
bind_rows(
as_draws_df(m11.4) |>
select(.draw, `a[1]`:`b[4]`) ,
as_draws_df(m11.6) |>
select(.draw, `a[1]`:`b[4]`)
) |>
mutate(type = rep(c("disaggregated", "aggregated"), each = n() / 2)) |>
pivot_longer(`a[1]`:`b[4]`) |>
mutate(class = str_extract(name, "\\w")) |>
ggplot(aes(x = value, y = name,
color = type)) +
stat_pointinterval(.width = 0.89, linewidth = 1, shape = 1,
position = position_dodge(width = -0.5)) +
scale_y_discrete(NULL, labels = ggplot2:::parse_safe) +
xlab("posterior") +
facet_wrap(~ class, scales = "free")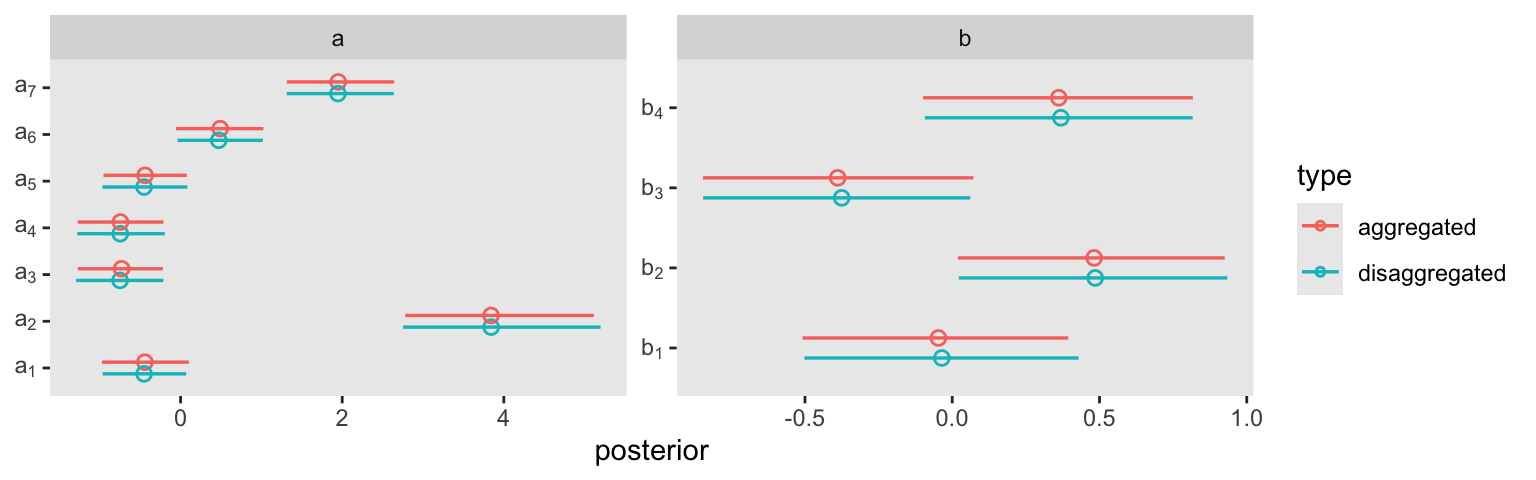
Different version of the likelihood, same model. Feed the extract_log_lik() results into loo(), and save output as loo_m11.6.
loo_m11.6 <- extract_log_lik(m11.6) |>
loo()Warning: Some Pareto k diagnostic values are too high. See help('pareto-k-diagnostic') for details.# What?
print(loo_m11.6)
Computed from 4000 by 28 log-likelihood matrix.
Estimate SE
elpd_loo -57.1 4.2
p_loo 8.4 1.5
looic 114.1 8.4
------
MCSE of elpd_loo is NA.
MCSE and ESS estimates assume independent draws (r_eff=1).
Pareto k diagnostic values:
Count Pct. Min. ESS
(-Inf, 0.7] (good) 27 96.4% 407
(0.7, 1] (bad) 1 3.6% <NA>
(1, Inf) (very bad) 0 0.0% <NA>
See help('pareto-k-diagnostic') for details.Unlike with McElreath’s compare() code in the text, the loo::loo_compare() will not let us directly compare the two versions of the model. I’m going to supress the output, but if you execute this code on your computer, you’ll see it returns a warning.
loo_compare(
extract_log_lik(m11.4) |> loo(),
loo_m11.6
) |>
print(simplify = FALSE)To understand what’s going on, consider how you might describe six 1’s out of nine trials in the aggregated form,
\[\Pr(6|9, p) = \frac{6!}{6!(9 - 6)!} p^6 (1 - p)^{9 - 6}.\]
If we still stick with the same data, but this time re-express those as nine dichotomous data points, we now describe their joint probability as
\[\Pr(1, 1, 1, 1, 1, 1, 0, 0, 0 \mid p) = p^6 (1 - p)^{9 - 6}.\]
Let’s work this out in code.
# Deviance of aggregated 6-in-9
-2 * dbinom(6, size = 9, prob = 0.2, log = TRUE)[1] 11.79048# Deviance of dis-aggregated
-2 * sum(dbinom(c(1, 1, 1, 1, 1, 1, 0, 0, 0), size = 1, prob = 0.2, log = TRUE))[1] 20.65212But this difference is entirely meaningless. It is just a side effect of how we organized the data. The posterior distribution for the probability of success on each trial will end up the same, either way. (p. 339)
This is what our coefficient plot showed us, above. The posterior distribution was the same within simulation variance for m11.4 and m11.6. Just like McElreath reported in the text, we also got a warning about high Pareto \(k\) values from the aggregated binomial model, m11.6. To access the message and its associated table directly, we can feed our loo_m11.6 object into the loo::pareto_k_table function.
loo_m11.6 |>
loo::pareto_k_table()Pareto k diagnostic values:
Count Pct. Min. ESS
(-Inf, 0.7] (good) 27 96.4% 407
(0.7, 1] (bad) 1 3.6% <NA>
(1, Inf) (very bad) 0 0.0% <NA> 11.1.4 Aggregated binomial: Graduate school admissions
Load the infamous UCBadmit data.
data(UCBadmit, package = "rethinking")
d <- UCBadmit
rm(UCBadmit)
# What?
print(d) dept applicant.gender admit reject applications
1 A male 512 313 825
2 A female 89 19 108
3 B male 353 207 560
4 B female 17 8 25
5 C male 120 205 325
6 C female 202 391 593
7 D male 138 279 417
8 D female 131 244 375
9 E male 53 138 191
10 E female 94 299 393
11 F male 22 351 373
12 F female 24 317 341Now compute our new index variable, gid. Notice that if we save gid as a factor, the compose_data() a couple blocks below will automatically compute n_gid. We’ll also slip in a case variable that saves the row numbers as a factor, which will come in handy later when we plot.
d <- d |>
mutate(gid = ifelse(applicant.gender == "male", 1, 2) |> factor(),
case = 1:n() |> factor())
# What?
d |>
distinct(applicant.gender, gid) applicant.gender gid
1 male 1
2 female 2The univariable logistic model with male as the sole predictor of admit follows the form
\[ \begin{align*} \text{admit}_i & \sim \operatorname{Binomial}(n_i, p_i) \\ \text{logit}(p_i) & = \alpha_{\text{gid}[i]} \\ \alpha_j & \sim \operatorname{Normal}(0, 1.5), \end{align*} \]
where \(n_i = \text{applications}_i\), the rows are indexed by \(i\), and the two levels of \(\text{gid}\) are indexed by \(j\).
Make the stan_data with the compose_data() function.
stan_data <- d |>
select(dept, gid, admit, applications) |>
compose_data()
# What?
str(stan_data)List of 7
$ dept : num [1:12(1d)] 1 1 2 2 3 3 4 4 5 5 ...
$ n_dept : int 6
$ gid : num [1:12(1d)] 1 2 1 2 1 2 1 2 1 2 ...
$ n_gid : int 2
$ admit : int [1:12(1d)] 512 89 353 17 120 202 138 131 53 94 ...
$ applications: int [1:12(1d)] 825 108 560 25 325 593 417 375 191 393 ...
$ n : int 12Make the model_code and fit m11.7 with stan(). Note the generated quantities block for the posterior-predictive check to come.
model_code_11.7 <- '
data {
int<lower=1> n;
int<lower=1> n_gid;
array[n] int gid;
array[n] int<lower=1> applications;
array[n] int<lower=0> admit;
}
parameters {
vector[n_gid] a;
}
model {
admit ~ binomial(applications, inv_logit(a[gid]));
a ~ normal(0, 1.5);
}
generated quantities {
// For the pp check
array[n] int<lower=0> pp_admit = binomial_rng(applications, inv_logit(a[gid]));
}
'
m11.7 <- stan(
model_code = model_code_11.7,
data = stan_data,
cores = 4, iter = 4000, seed = 11)Check the model summary.
print(m11.7, pars = "a", probs = c(0.055, 0.945))Inference for Stan model: anon_model.
4 chains, each with iter=4000; warmup=2000; thin=1;
post-warmup draws per chain=2000, total post-warmup draws=8000.
mean se_mean sd 5.5% 94.5% n_eff Rhat
a[1] -0.22 0 0.04 -0.28 -0.16 5765 1
a[2] -0.83 0 0.05 -0.91 -0.75 5699 1
Samples were drawn using NUTS(diag_e) at Thu Aug 15 11:33:46 2024.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).We’ll hold off on computing diff_a and diff_p, for a moment, and jump straight to Figure 11.5. Note how we’re using the pp_admit values we computed with the generated quantities block.
m11.7 |>
spread_draws(pp_admit[i]) |>
left_join(d |>
mutate(i = as.integer(case)),
by = join_by(i)) |>
ggplot(aes(x = gid)) +
stat_pointinterval(aes(y = pp_admit / applications),
.width = 0.89, linewidth = 1, shape = 1) +
geom_point(data = d,
aes(y = admit / applications),
color = "blue") +
geom_path(data = d,
aes(y = admit / applications, group = dept),
color = "blue") +
scale_x_discrete(NULL, labels = c("male", "female")) +
scale_y_continuous("admit", limits = 0:1) +
facet_wrap(~ dept, nrow = 1)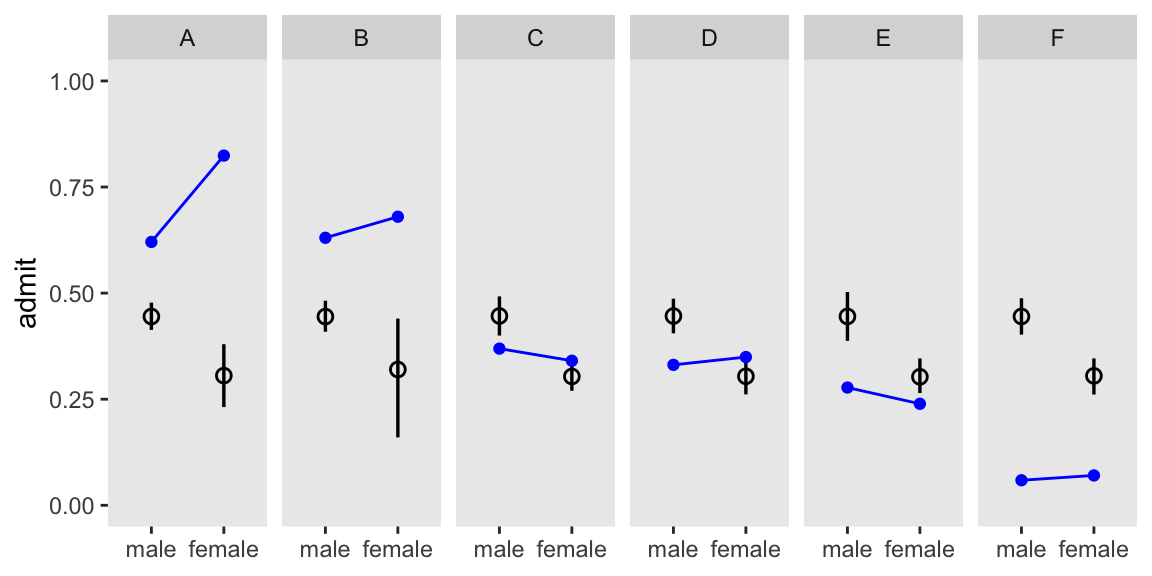
I should acknowledge I got the idea to reformat the plot this way from Arel-Bundock’s work.
Now we fit the model
\[ \begin{align*} \text{admit}_i & \sim \operatorname{Binomial} (n_i, p_i) \\ \text{logit}(p_i) & = \alpha_{\text{gid}[i]} + \delta_{\text{dept}[i]} \\ \alpha_j & \sim \operatorname{Normal} (0, 1.5) \\ \delta_k & \sim \operatorname{Normal} (0, 1.5), \end{align*} \]
where departments are indexed by \(k\). Make the model_code and fit m11.8 with stan(). Note how we’re increasing the iter argument in stan().
model_code_11.8 <- '
data {
int<lower=1> n;
int<lower=1> n_gid;
int<lower=1> n_dept;
array[n] int gid;
array[n] int dept;
array[n] int applications;
array[n] int<lower=0> admit;
}
parameters {
vector[n_gid] a;
vector[n_dept] d;
}
model {
admit ~ binomial(applications, inv_logit(a[gid] + d[dept]));
a ~ normal(0, 1.5);
d ~ normal(0, 1.5);
}
generated quantities {
// For the pp check
array[n] int<lower=0> pp_admit = binomial_rng(applications, inv_logit(a[gid] + d[dept]));
}
'
m11.8 <- stan(
model_code = model_code_11.8,
data = stan_data,
cores = 4, iter = 4000, seed = 11)Check the model summary.
print(m11.8, pars = c("a", "d"), probs = c(0.055, 0.945))Inference for Stan model: anon_model.
4 chains, each with iter=4000; warmup=2000; thin=1;
post-warmup draws per chain=2000, total post-warmup draws=8000.
mean se_mean sd 5.5% 94.5% n_eff Rhat
a[1] -0.54 0.02 0.54 -1.42 0.34 603 1
a[2] -0.45 0.02 0.54 -1.33 0.44 607 1
d[1] 1.12 0.02 0.54 0.24 2.00 604 1
d[2] 1.08 0.02 0.55 0.18 1.96 603 1
d[3] -0.14 0.02 0.54 -1.02 0.74 611 1
d[4] -0.17 0.02 0.55 -1.05 0.71 610 1
d[5] -0.61 0.02 0.55 -1.51 0.28 623 1
d[6] -2.17 0.02 0.55 -3.07 -1.27 642 1
Samples were drawn using NUTS(diag_e) at Thu Aug 15 11:35:07 2024.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).Here we’ll show the contrasts for the two models in a coefficient plot.
bind_rows(
as_draws_df(m11.7) |> select(.draw, `a[1]`:`a[2]`),
as_draws_df(m11.8) |> select(.draw, `a[1]`:`a[2]`)
) |>
mutate(fit = rep(c("m11.7", "m11.8"), each = n() / 2),
diff_a = `a[1]` - `a[2]`,
diff_p = plogis(`a[1]`) - plogis(`a[2]`)) |>
pivot_longer(contains("diff")) |>
ggplot(aes(x = value, y = name,
color = fit)) +
geom_vline(xintercept = 0, color = "white") +
stat_pointinterval(.width = 0.89, linewidth = 1, shape = 1,
position = position_dodge(width = -0.5)) +
labs(x = "contrast",
y = NULL)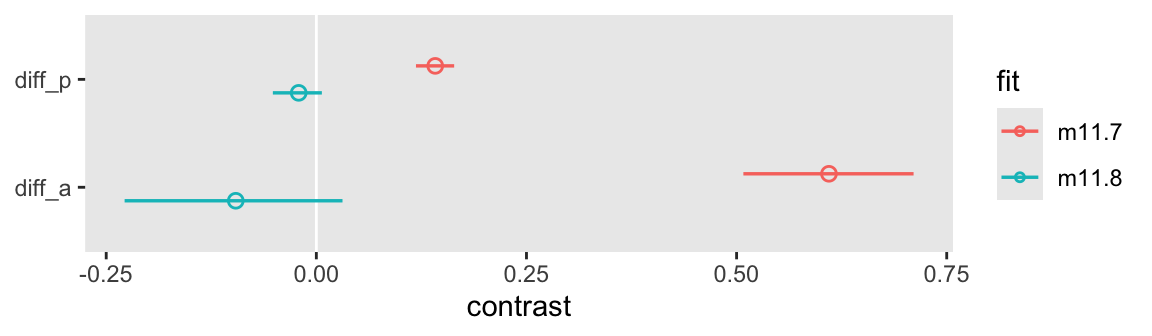
Here’s our tidyverse-style tabulation of the proportions of applicants in each department by gid.
d |>
group_by(dept) |>
mutate(proportion = applications / sum(applications)) |>
select(dept, gid, proportion) |>
pivot_wider(names_from = dept,
values_from = proportion) |>
mutate_if(is.double, round, digits = 2)# A tibble: 2 × 7
gid A B C D E F
<fct> <dbl> <dbl> <dbl> <dbl> <dbl> <dbl>
1 1 0.88 0.96 0.35 0.53 0.33 0.52
2 2 0.12 0.04 0.65 0.47 0.67 0.48If we make another version of Figure 11.5 with m11.8, we’ll see conditioning on both substantially improved the posterior predictive distribution.
m11.8 |>
spread_draws(pp_admit[i]) |>
left_join(d |>
mutate(i = as.integer(case)),
by = join_by(i)) |>
ggplot(aes(x = gid)) +
stat_pointinterval(aes(y = pp_admit / applications),
.width = 0.89, linewidth = 1, shape = 1) +
geom_point(data = d,
aes(y = admit / applications),
color = "blue") +
geom_path(data = d,
aes(y = admit / applications, group = dept),
color = "blue") +
scale_x_discrete(NULL, labels = c("male", "female")) +
scale_y_continuous("admit", limits = 0:1) +
facet_wrap(~ dept, nrow = 1)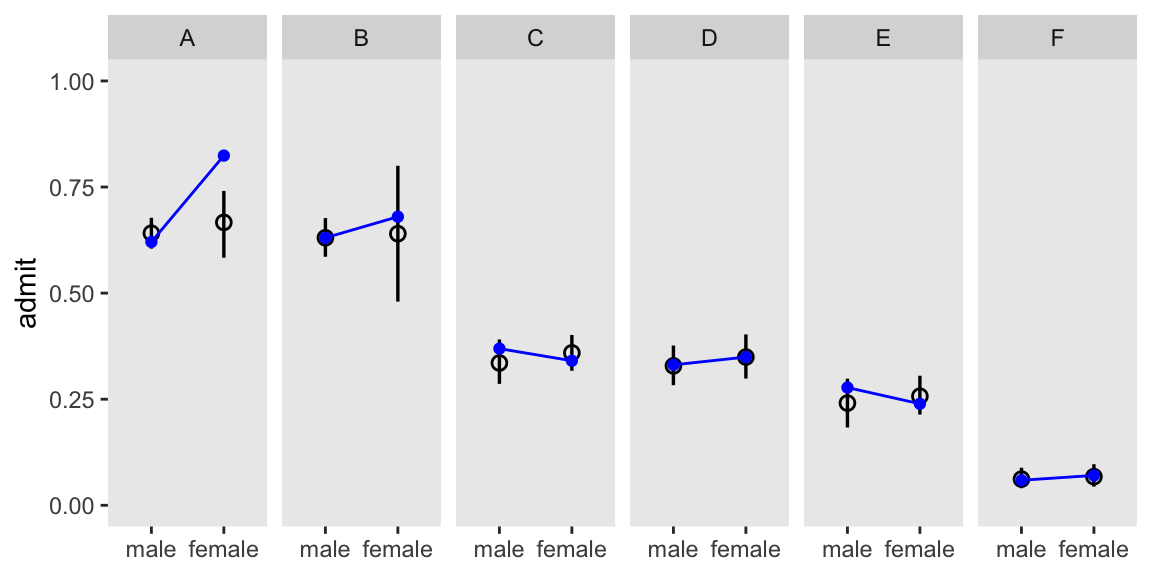
McElreath recommended we look at the pairs() plot to get a sense of how highly correlated the parameters in our m11.8 model are. Here’s the plot.
pairs(m11.8, pars = c("a", "d"), gap = 0.25)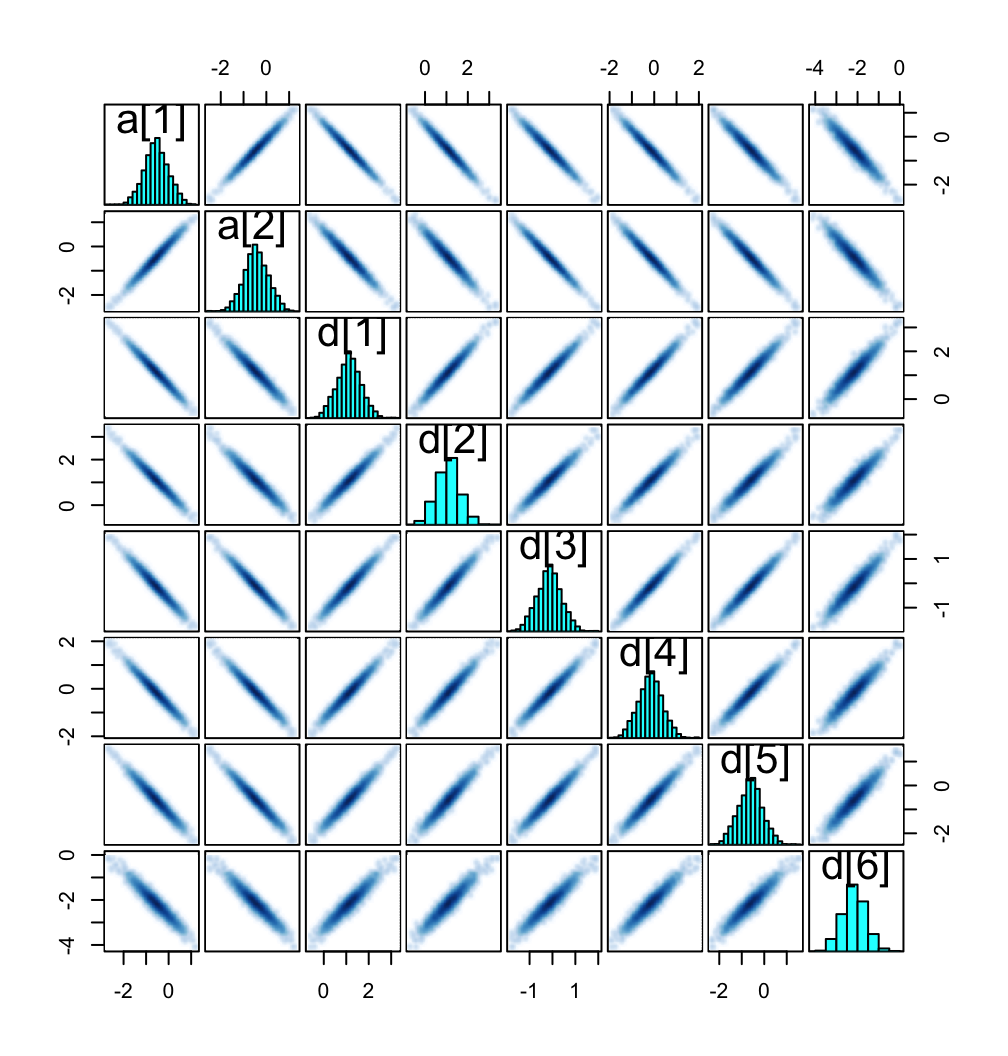
11.1.4.1 Rethinking: Simpson’s paradox is not a paradox
11.2 Poisson regression
11.2.1 Example: Oceanic tool complexity
Load the Kline data (see Kline & Boyd, 2010).
data(Kline, package = "rethinking")
d <- Kline
rm(Kline)
# What?
print(d) culture population contact total_tools mean_TU
1 Malekula 1100 low 13 3.2
2 Tikopia 1500 low 22 4.7
3 Santa Cruz 3600 low 24 4.0
4 Yap 4791 high 43 5.0
5 Lau Fiji 7400 high 33 5.0
6 Trobriand 8000 high 19 4.0
7 Chuuk 9200 high 40 3.8
8 Manus 13000 low 28 6.6
9 Tonga 17500 high 55 5.4
10 Hawaii 275000 low 71 6.6Here are our new columns.
d <- d |>
mutate(log_pop_std = (log(population) - mean(log(population))) / sd(log(population)),
cid = ifelse(contact == "high", "2", "1"))Our statistical model will follow the form
\[ \begin{align*} \text{total-tools}_i & \sim \operatorname{Poisson}(\lambda_i) \\ \log(\lambda_i) & = \alpha_{\text{cid}[i]} + \beta_{\text{cid}[i]} \text{log-pop-std}_i \\ \alpha_j & \sim \; ? \\ \beta_j & \sim \; ?, \end{align*} \]
where the priors for \(\alpha_j\) and \(\beta_j\) have yet be defined. If we continue our convention of using a Normal prior on the \(\alpha\) parameters, we should recognize those will be log-Normal distributed on the outcome scale. Why? Because we’re modeling \(\lambda\) with the log link. Here’s our version of Figure 11.7, depicting the two log-Normal priors considered in the text.
d_plot <- tibble(
x = c(3, 22),
y = c(0.055, 0.04),
meanlog = c(0, 3),
sdlog = c(10, 0.5)) |>
expand_grid(number = seq(from = 0, to = 100, length.out = 200)) |>
mutate(density = dlnorm(number, meanlog, sdlog),
group = str_c("alpha%~%Normal(", meanlog, ", ", sdlog, ")"))
d_plot |>
ggplot(aes(fill = group, color = group)) +
geom_area(aes(x = number, y = density),
alpha = 3/4, linewidth = 0, position = "identity") +
geom_text(data = d_plot |>
group_by(group) |>
slice(1),
aes(x = x, y = y, label = group),
hjust = 0, parse = TRUE) +
scale_y_continuous(NULL, breaks = NULL) +
xlab("mean number of tools") +
theme(legend.position = "none")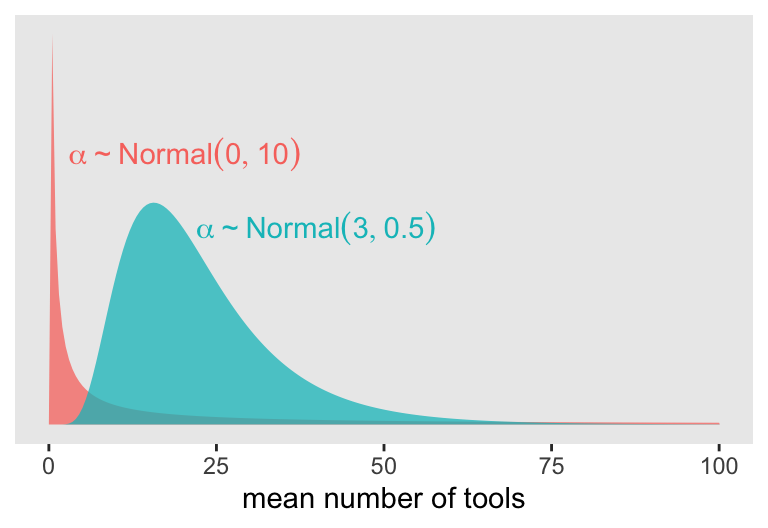
In this context, \(\alpha \sim \operatorname{Normal}(0, 10)\) has a very long tail on the outcome scale. The mean of the log-Normal distribution, recall, is \(\exp (\mu + \sigma^2/2)\). Here that is in code.
exp(0 + 10^2 / 2)[1] 5.184706e+21That is very large. Here’s the same thing in a simulation.
set.seed(11)
rnorm(1e4, 0, 10) |>
exp() |>
mean()[1] 1.61276e+12Now compute the mean for the other prior under consideration, \(\alpha \sim \operatorname{Normal}(3, 0.5)\).
exp(3 + 0.5^2 / 2)[1] 22.7599This is much smaller and more reasonable.
Now let’s prepare to make the top row of Figure 11.8. In this portion of the figure, we consider the implications of two competing priors for \(\beta\) while holding the prior for \(\alpha\) at \(\operatorname{Normal}(3, 0.5)\). The two \(\beta\) priors under consideration are \(\operatorname{Normal}(0, 10)\) and \(\operatorname{Normal}(0, 0.2)\).
set.seed(11)
# How many lines would you like?
n <- 100
# Simulate and wrangle
tibble(i = 1:n,
a = rnorm(n, mean = 3, sd = 0.5)) |>
mutate(`beta%~%Normal(0*', '*10)` = rnorm(n, mean = 0 , sd = 10),
`beta%~%Normal(0*', '*0.2)` = rnorm(n, mean = 0 , sd = 0.2)) |>
pivot_longer(contains("beta"),
values_to = "b",
names_to = "prior") |>
expand_grid(x = seq(from = -2, to = 2, length.out = 100)) |>
mutate(prior = fct_rev(prior)) |>
# Plot
ggplot(aes(x = x, y = exp(a + b * x), group = i)) +
geom_line(alpha = 2/3, linewidth = 1/4) +
labs(x = "log population (std)",
y = "total tools") +
coord_cartesian(ylim = c(0, 100)) +
facet_wrap(~ prior, labeller = label_parsed)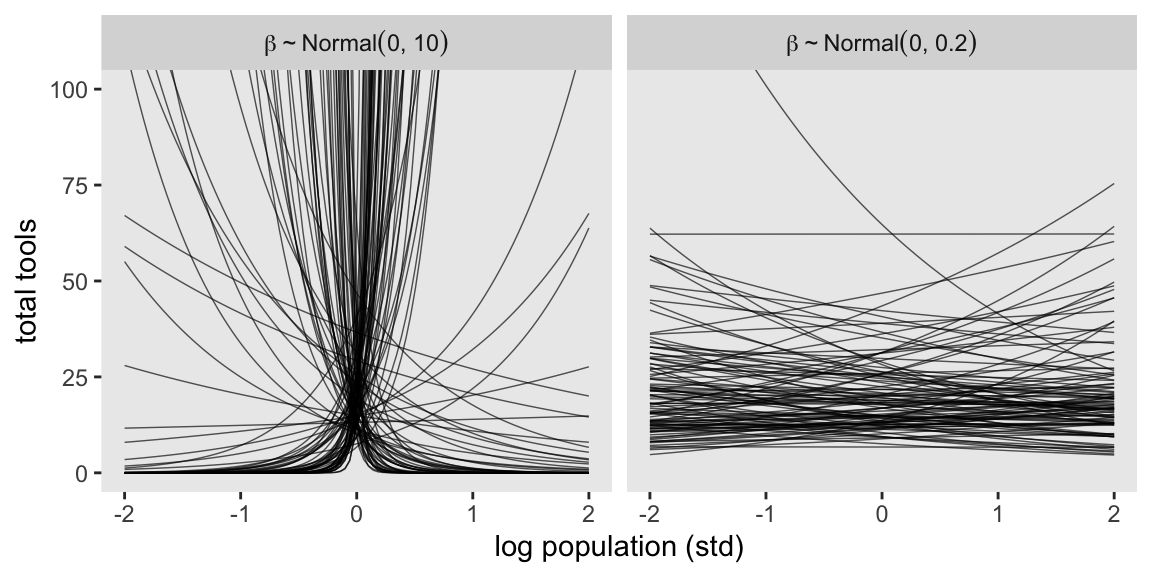
It turns out that many of the lines considered plausible under \(\operatorname{Normal}(0, 10)\) are disturbingly extreme. Here is what \(\alpha \sim \operatorname{Normal}(3, 0.5)\) and \(\beta \sim \operatorname{Normal}(0, 0.2)\) would mean when the \(x\)-axis is on the log population scale and the population scale.
set.seed(11)
prior <- tibble(
i = 1:n,
a = rnorm(n, mean = 3, sd = 0.5),
b = rnorm(n, mean = 0, sd = 0.2)) |>
expand_grid(x = seq(from = log(100), to = log(200000), length.out = 100))
# Left
p1 <- prior |>
ggplot(aes(x = x, y = exp(a + b * x), group = i)) +
geom_line(alpha = 2/3, linewidth = 1/4) +
labs(x = "log population",
y = "total tools") +
coord_cartesian(xlim = c(log(100), log(200000)),
ylim = c(0, 500))
# Right
p2 <- prior |>
ggplot(aes(x = exp(x), y = exp(a + b * x), group = i)) +
geom_line(alpha = 2/3, linewidth = 1/4) +
scale_y_continuous(NULL, breaks = NULL) +
xlab("population") +
coord_cartesian(xlim = c(100, 200000),
ylim = c(0, 500))
# Combine, add facet strips, and display
(p1 | p2) &
facet_wrap(~ "atop(alpha%~%Normal(3*', '*0.5), beta%~%Normal(0*', '*0.2))",
labeller = label_parsed)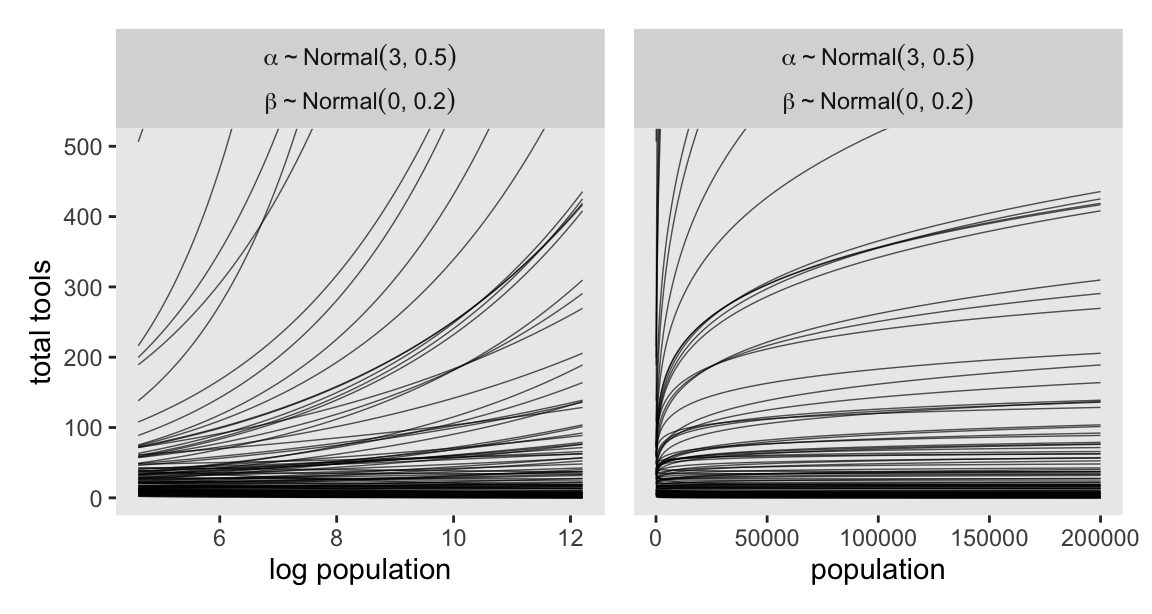
Okay, after settling on our two priors, the updated model formula is
\[ \begin{align*} y_i & \sim \operatorname{Poisson}(\lambda_i) \\ \log(\lambda_i) & = \alpha + \beta (x_i - \bar x) \\ \alpha & \sim \operatorname{Normal}(3, 0.5) \\ \beta & \sim \operatorname{Normal}(0, 0.2). \end{align*} \]
We’re finally ready to start fitting the models. First, define the stan_data.
stan_data <- d |>
select(total_tools, log_pop_std, cid) |>
compose_data()
# What?
str(stan_data)List of 5
$ total_tools: int [1:10(1d)] 13 22 24 43 33 19 40 28 55 71
$ log_pop_std: num [1:10(1d)] -1.2915 -1.0886 -0.5158 -0.3288 -0.0443 ...
$ cid : num [1:10(1d)] 1 1 1 2 2 2 2 1 2 1
$ n_cid : int 2
$ n : int 10Define model_code_11.9 and model_code_11.10.
# Intercept only
model_code_11.9 <- '
data {
int<lower=1> n;
array[n] int<lower=0> total_tools;
}
parameters {
real a;
}
model {
total_tools ~ poisson(exp(a));
a ~ normal(3, 0.5);
}
generated quantities {
vector[n] log_lik;
for (i in 1:n) log_lik[i] = poisson_lpmf(total_tools[i] | exp(a));
}
'
# Interaction model
model_code_11.10 <- '
data {
int<lower=1> n;
int<lower=1> n_cid;
array[n] int cid;
vector[n] log_pop_std;
array[n] int<lower=0> total_tools;
}
parameters {
vector[n_cid] a;
vector[n_cid] b;
}
model {
vector[n] lambda;
lambda = exp(a[cid] + b[cid] .* log_pop_std);
total_tools ~ poisson(lambda);
a ~ normal(3, 0.5);
b ~ normal(0, 0.2);
}
generated quantities {
vector[n] log_lik;
for (i in 1:n) log_lik[i] = poisson_lpmf(total_tools[i] | exp(a[cid[i]] + b[cid[i]] .* log_pop_std[i]));
}
'As we discussed back in Section 1.3.3, we also could have fit these models with the poisson_log() function in place of poisson(), and computed the log_lik values with the poisson_log_lpmf() function in place of poisson_lpmf(). Those functions would have obviated our use of the exp() function for the linear model. Consider fitting the model both ways to see which is faster or returns better HMC diagnostics.
For now, we sample from the two posteriors as they’ve been currently defined with stan().
m11.9 <- stan(
data = stan_data,
model_code = model_code_11.9,
cores = 4, seed = 11)
m11.10 <- stan(
data = stan_data,
model_code = model_code_11.10,
cores = 4, seed = 11)Compare the two models by the LOO.
l11.9 <- extract_log_lik(m11.9) |> loo()
l11.10 <- extract_log_lik(m11.10) |> loo()Warning: Some Pareto k diagnostic values are too high. See help('pareto-k-diagnostic') for details.loo_compare(l11.9, l11.10) |>
print(simplify = FALSE) elpd_diff se_diff elpd_loo se_elpd_loo p_loo se_p_loo looic se_looic
model2 0.0 0.0 -42.6 6.6 6.9 2.6 85.2 13.2
model1 -28.1 16.4 -70.7 16.7 8.3 3.5 141.4 33.4 Like McElreath reported in the text, we have a Pareto-\(k\) warning. We can inspect the \(k\) values with loo::pareto_k_table().
pareto_k_table(l11.10)Pareto k diagnostic values:
Count Pct. Min. ESS
(-Inf, 0.7] (good) 8 80.0% 344
(0.7, 1] (bad) 1 10.0% <NA>
(1, Inf) (very bad) 1 10.0% <NA> Let’s take a closer look.
d |>
select(culture) |>
mutate(k = l11.10$diagnostics$pareto_k) |>
arrange(desc(k)) |>
mutate_if(is.double, round, digits = 2) culture k
1 Hawaii 1.02
2 Tonga 0.83
3 Trobriand 0.56
4 Yap 0.55
5 Malekula 0.42
6 Manus 0.32
7 Tikopia 0.26
8 Lau Fiji 0.25
9 Santa Cruz 0.20
10 Chuuk 0.13It turns out Hawaii is very influential. Figure 11.9 will clarify why. For a little practice while computing the trajectories, we’ll use an as_draws_df()-based workflow for the panel on the left, and use a spread_draws()-based workflow for the panel on the right.
# For subsetting the labels in the left panel
culture_vec <- c("Hawaii", "Tonga", "Trobriand", "Yap")
# Left
p1 <- as_draws_df(m11.10) |>
select(.draw, `a[1]`:`b[2]`) |>
pivot_longer(-.draw) |>
mutate(parameter = str_extract(name, "[a-z]"),
cid = str_extract(name, "\\d")) |>
select(-name) |>
pivot_wider(names_from = parameter, values_from = value) |>
expand_grid(log_pop_std = seq(from = -4.5, to = 2.5, length.out = 100)) |>
mutate(total_tools = exp(a + b * log_pop_std)) |>
ggplot(aes(x = log_pop_std, y = total_tools,
color = cid, fill = cid, group = cid)) +
stat_lineribbon(.width = 0.89, alpha = 1/4) +
geom_point(data = d |>
mutate(k = l11.10$diagnostics$pareto_k),
aes(size = k)) +
geom_text(data = d |>
mutate(k = l11.10$diagnostics$pareto_k) |>
mutate(label = str_c(culture, " (", round(k, digits = 2), ")"),
vjust = ifelse(total_tools > 30, -0.3, 1.3)) |>
filter(culture %in% culture_vec),
aes(label = label, vjust = vjust),
hjust = 1.1, size = 3) +
labs(x = "log population (std)",
y = "total tools") +
coord_cartesian(xlim = range(d$log_pop_std),
ylim = c(0, 80))
# Right
p2 <- spread_draws(m11.10, a[cid], b[cid]) |>
mutate(cid = as.character(cid)) |>
expand_grid(log_pop_std = seq(from = -4.5, to = 2.5, length.out = 100)) |>
mutate(total_tools = exp(a + b * log_pop_std)) |>
mutate(population = exp((log_pop_std * sd(log(d$population))) + mean(log(d$population)))) |>
ggplot(aes(x = population, y = total_tools,
color = cid, fill = cid, group = cid)) +
stat_lineribbon(.width = 0.89, alpha = 1/4) +
geom_point(data = d |>
mutate(k = l11.10$diagnostics$pareto_k),
aes(size = k)) +
scale_x_continuous("population", breaks = c(0, 50000, 150000, 250000),
labels = scales::comma(c(0, 50000, 150000, 250000))) +
scale_y_continuous(NULL, breaks = NULL) +
coord_cartesian(xlim = range(d$population),
ylim = c(0, 80))
# Combine, adjust, and display
(p1 | p2) &
scale_color_viridis_d(option = "A", end = 0.6) &
scale_fill_viridis_d(option = "A", end = 0.6) &
scale_size_continuous(range = c(0.1, 5), limits = c(0.1, 1.1)) &
theme(legend.position = "none")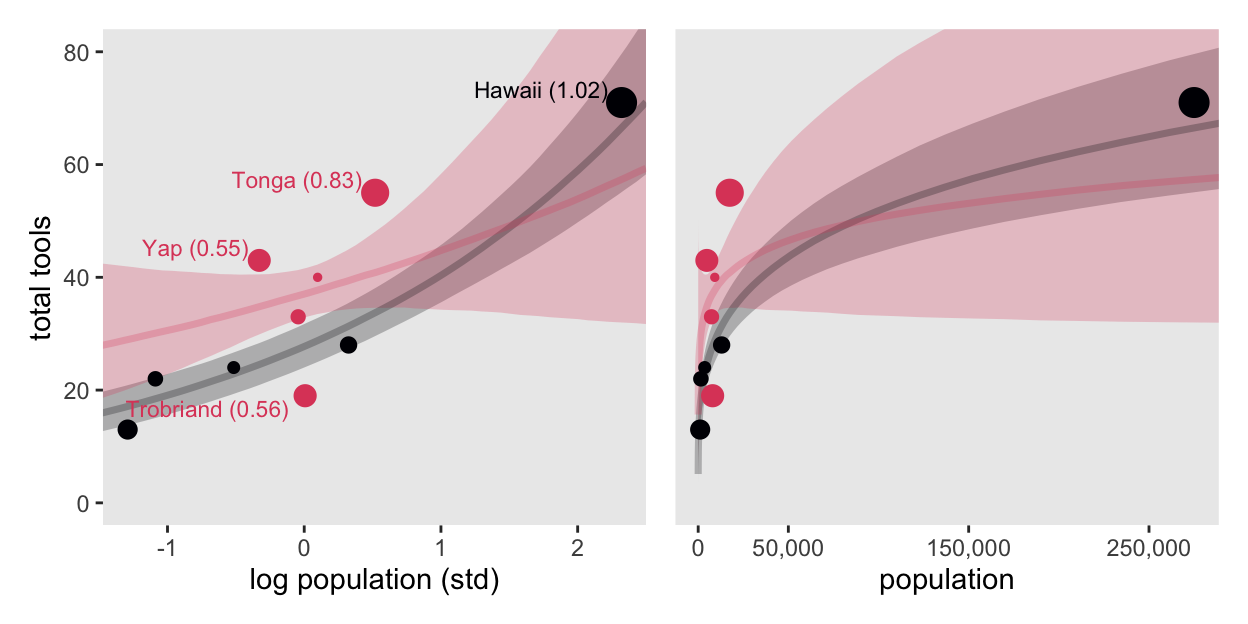
Hawaii is influential in that it has a very large population relative to the other islands.
11.2.1.1 Overthinking: Modeling tool innovation
McElreath’s theoretical, or scientific, model for total_tools is
\[\widehat{\text{total-tools}} = \frac{\alpha_{\text{cid}[i]} \: \text{population}^{\beta_{\text{cid}[i]}}}{\gamma}.\]
We can use the Poisson likelihood to express this in a Bayesian model as
\[ \begin{align*} \text{total-tools} & \sim \operatorname{Poisson}(\lambda_i) \\ \lambda_i & = \left[ \exp (\alpha_{\text{cid}[i]}) \text{population}_i^{\beta_{\text{cid}[i]}} \right] / \gamma \\ \alpha_j & \sim \operatorname{Normal}(1, 1) \\ \beta_j & \sim \operatorname{Exponential}(1) \\ \gamma & \sim \operatorname{Exponential}(1), \end{align*} \]
where we exponentiate \(\alpha_{\text{cid}[i]}\) to restrict the posterior to zero and above. To fit that model with rstan, first we make some data d_pred for the predictions we’ll display in the plot.
d_pred <- tibble(
population = seq(from = 0, to = 3e5, length.out = 101) |>
as.integer()) |>
expand_grid(cid = 1:2) |>
mutate(i = 1:n())
# What?
glimpse(d_pred)Rows: 202
Columns: 3
$ population <int> 0, 0, 3000, 3000, 6000, 6000, 9000, 9000, 12000, 12000, 150…
$ cid <int> 1, 2, 1, 2, 1, 2, 1, 2, 1, 2, 1, 2, 1, 2, 1, 2, 1, 2, 1, 2,…
$ i <int> 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, …Now make the stan_data.
stan_data <- d |>
mutate(population = as.double(population)) |>
select(total_tools, population, cid) |>
compose_data(population_pred = d_pred$population,
cid_pred = d_pred$cid,
n_pred = nrow(d_pred))
# What?
str(stan_data)List of 8
$ total_tools : int [1:10(1d)] 13 22 24 43 33 19 40 28 55 71
$ population : num [1:10(1d)] 1100 1500 3600 4791 7400 ...
$ cid : num [1:10(1d)] 1 1 1 2 2 2 2 1 2 1
$ n_cid : int 2
$ n : int 10
$ population_pred: int [1:202] 0 0 3000 3000 6000 6000 9000 9000 12000 12000 ...
$ cid_pred : int [1:202] 1 2 1 2 1 2 1 2 1 2 ...
$ n_pred : int 202Define model_code_11.11.
model_code_11.11 <- '
data {
int<lower=1> n;
int<lower=1> n_cid;
array[n] int cid;
vector[n] population;
array[n] int<lower=0> total_tools;
// For predictions
int<lower=1> n_pred;
array[n_pred] int cid_pred;
vector[n_pred] population_pred;
}
parameters {
vector[n_cid] a;
vector<lower=0>[n_cid] b;
real<lower=0> g;
}
transformed parameters {
vector[n] lambda;
lambda = exp(a[cid]) .* population^b[cid] / g;
}
model {
total_tools ~ poisson(lambda);
a ~ normal(1, 1);
b ~ exponential(1);
g ~ exponential(1);
}
generated quantities {
vector[n] log_lik;
vector[n_pred] lambda_pred;
for (i in 1:n) log_lik[i] = poisson_lpmf(total_tools[i] | lambda[i]);
lambda_pred = exp(a[cid_pred]) .* population_pred^b[cid_pred] / g;
}
'Sample from the posterior.
m11.11 <- stan(
data = stan_data,
model_code = model_code_11.11,
cores = 4, seed = 11)Check the model summary.
print(m11.11, pars = c("a", "b", "g"), probs = c(0.055, 0.945))Inference for Stan model: anon_model.
4 chains, each with iter=2000; warmup=1000; thin=1;
post-warmup draws per chain=1000, total post-warmup draws=4000.
mean se_mean sd 5.5% 94.5% n_eff Rhat
a[1] 0.92 0.02 0.68 -0.22 1.97 1383 1
a[2] 0.92 0.02 0.83 -0.42 2.26 1967 1
b[1] 0.26 0.00 0.03 0.20 0.32 1964 1
b[2] 0.29 0.00 0.11 0.12 0.46 1480 1
g 1.15 0.02 0.76 0.30 2.48 1605 1
Samples were drawn using NUTS(diag_e) at Thu Aug 15 11:44:42 2024.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).Compute and check the PSIS-LOO estimates along with their diagnostic Pareto-\(k\) values.
l11.11 <- extract_log_lik(m11.11) |> loo()Warning: Some Pareto k diagnostic values are too high. See help('pareto-k-diagnostic') for details.print(l11.11)
Computed from 4000 by 10 log-likelihood matrix.
Estimate SE
elpd_loo -40.6 6.0
p_loo 5.5 1.9
looic 81.3 12.0
------
MCSE of elpd_loo is NA.
MCSE and ESS estimates assume independent draws (r_eff=1).
Pareto k diagnostic values:
Count Pct. Min. ESS
(-Inf, 0.7] (good) 9 90.0% 274
(0.7, 1] (bad) 1 10.0% <NA>
(1, Inf) (very bad) 0 0.0% <NA>
See help('pareto-k-diagnostic') for details.We still have one high Pareto-\(k\) value. Recall that due to the very small sample size, this isn’t entirely surprising. If you’re curious, here’s a scatter plot of the Pareto-\(k\) values for this model and the last.
tibble(m11.10 = l11.10$diagnostics$pareto_k,
m11.11 = l11.11$diagnostics$pareto_k) |>
ggplot(aes(x = m11.10, y = m11.11)) +
geom_abline(color = "white") +
geom_point() +
scale_x_continuous(breaks = 0:5 / 5 + 0.1, limits = c(0.1, 1.1)) +
scale_y_continuous(breaks = 0:5 / 5 + 0.1, limits = c(0.1, 1.1)) +
labs(subtitle = expression(Pareto~italic(k)))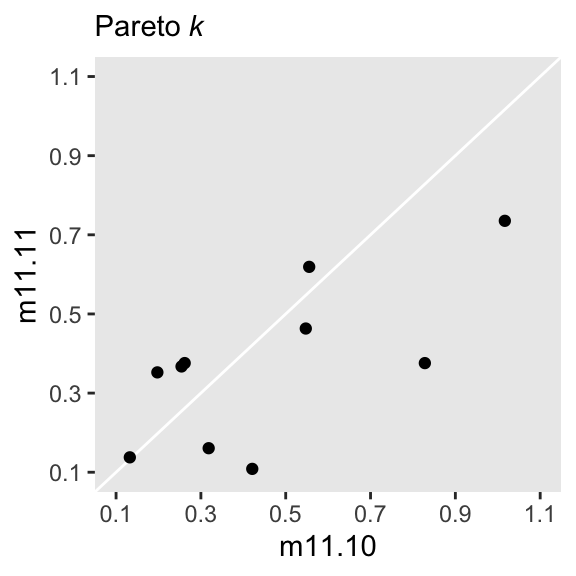
Okay, it’s time to make Figure 11.10. In the first line in the Wrangle section below, note how we’re using spread_draws() to extract the lambda_pred values we defined in the generated quantities, above.
# For the annotation
d_text <- distinct(d, cid, contact) |>
mutate(population = c(210000, 72500),
total_tools = c(59, 68),
label = str_c(contact, " contact"))
# Wrangle
spread_draws(m11.11, lambda_pred[i]) |>
left_join(d_pred, by = join_by(i)) |>
mutate(cid = as.character(cid)) |>
rename(total_tools = lambda_pred) |>
# Plot!
ggplot(aes(x = population, y = total_tools,
color = cid, fill = cid, group = cid)) +
stat_lineribbon(.width = 0.89, alpha = 1/4) +
geom_point(data = d |>
mutate(k = l11.11$diagnostics$pareto_k),
aes(size = k)) +
geom_text(data = d_text,
aes(label = label)) +
scale_x_continuous("population", breaks = c(0, 50000, 150000, 250000),
labels = scales::comma(c(0, 50000, 150000, 250000))) +
scale_color_viridis_d(option = "A", end = 0.6) +
scale_fill_viridis_d(option = "A", end = 0.6) +
scale_size_continuous(range = c(0.1, 5), limits = c(0.1, 1)) +
ylab("total tools") +
coord_cartesian(xlim = range(d$population),
ylim = c(0, 80)) +
theme(legend.position = "none")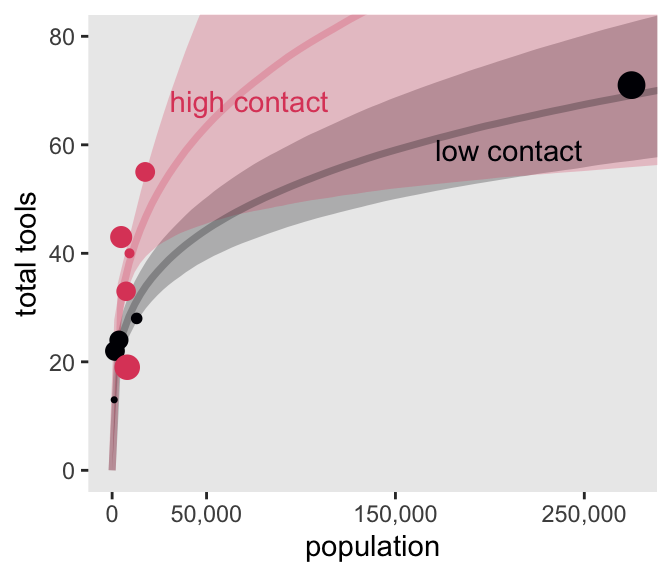
In case you were curious, here are the results if we compare m11.10 and m11.11 by the PSIS-LOO.
loo_compare(l11.10, l11.11) |>
print(simplify = FALSE) elpd_diff se_diff elpd_loo se_elpd_loo p_loo se_p_loo looic se_looic
model2 0.0 0.0 -40.6 6.0 5.5 1.9 81.3 12.0
model1 -2.0 2.9 -42.6 6.6 6.9 2.6 85.2 13.2 11.2.2 Negative binomial (gamma-Poisson) models
11.2.3 Example: Exposure and the offset
Here we simulate our industrious monk data.
set.seed(11)
num_days <- 30
y <- rpois(num_days, lambda = 1.5)
num_weeks <- 4
y_new <- rpois(num_weeks, lambda = 0.5 * 7)Now tidy the data and add log_days.
d <- tibble(
y = c(y, y_new),
days = rep(c(1, 7), times = c(num_days, num_weeks)), # this is the exposure
monastery = rep(0:1, times = c(num_days, num_weeks))) |>
mutate(log_days = log(days))
# What?
glimpse(d)Rows: 34
Columns: 4
$ y <int> 1, 0, 1, 0, 0, 4, 0, 1, 3, 0, 0, 1, 3, 3, 2, 2, 1, 1, 0, 1, …
$ days <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, …
$ monastery <int> 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, …
$ log_days <dbl> 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, 0, …Within the context of the Poisson likelihood, we can decompose \(\lambda\) into two parts, \(\mu\) (mean) and \(\tau\) (exposure), like this:
\[ y_i \sim \operatorname{Poisson}(\lambda_i) \\ \log \lambda_i = \log \frac{\mu_i}{\tau_i} = \log \mu_i - \log \tau_i. \]
Therefore, you can rewrite the equation if the exposure (\(\tau\)) varies in your data and you still want to model the mean (\(\mu\)). Using the model we’re about to fit as an example, here’s what that might look like:
\[ \begin{align*} y_i & \sim \operatorname{Poisson}(\mu_i) \\ \log \mu_i & = \color{blue}{\log \tau_i} + \alpha + \beta \text{monastery}_i \\ \alpha & \sim \operatorname{Normal}(0, 1) \\ \beta & \sim \operatorname{Normal}(0, 1), \end{align*} \]
where the offset \(\log \tau_i\) does not get a prior. In this context, its value is added directly to the right side of the formula. With the rstan package, we do this directly in the model block. First, though, let’s make the stan_data.
stan_data <- d |>
compose_data()
# What?
str(stan_data)List of 5
$ y : int [1:34(1d)] 1 0 1 0 0 4 0 1 3 0 ...
$ days : num [1:34(1d)] 1 1 1 1 1 1 1 1 1 1 ...
$ monastery: int [1:34(1d)] 0 0 0 0 0 0 0 0 0 0 ...
$ log_days : num [1:34(1d)] 0 0 0 0 0 0 0 0 0 0 ...
$ n : int 34Define model_code_11.12. Note how log_days does not get a coefficient in the lambda formula. It stands on its own.
model_code_11.12 <- '
data {
int<lower=1> n;
vector[n] log_days;
vector[n] monastery;
array[n] int<lower=0> y;
}
parameters {
real a;
real b;
}
model {
y ~ poisson(exp(log_days + a + b * monastery));
a ~ normal(0, 1);
b ~ normal(0, 1);
}
'Sample from the posterior.
m11.12 <- stan(
data = stan_data,
model_code = model_code_11.12,
cores = 4, seed = 11)As we look at the model summary, keep in mind that the parameters are on the per-one-unit-of-time scale. Since we simulated the data based on summary information from two units of time–one day and seven days–, this means the parameters are in the scale of \(\log (\lambda)\) per one day.
print(m11.12, probs = c(0.055, 0.945))Inference for Stan model: anon_model.
4 chains, each with iter=2000; warmup=1000; thin=1;
post-warmup draws per chain=1000, total post-warmup draws=4000.
mean se_mean sd 5.5% 94.5% n_eff Rhat
a -0.01 0.00 0.18 -0.31 0.26 1776 1
b -0.88 0.01 0.33 -1.40 -0.37 1861 1
lp__ -31.30 0.03 1.07 -33.31 -30.32 1639 1
Samples were drawn using NUTS(diag_e) at Thu Aug 15 11:46:33 2024.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).To get the posterior distributions for average daily outputs for the old and new monasteries, respectively, we’ll use use the formulas
\[ \begin{align*} \lambda_\text{old} & = \exp (\alpha) \;\;\; \text{and} \\ \lambda_\text{new} & = \exp (\alpha + \beta_\text{monastery}). \end{align*} \]
Following those transformations, we’ll summarize the \(\lambda\) distributions with medians and 89% HDIs with help from the tidybayes::mean_hdi() function.
as_draws_df(m11.12) |>
mutate(lambda_old = exp(a),
lambda_new = exp(a + b)) |>
pivot_longer(contains("lambda")) |>
mutate(name = factor(name, levels = c("lambda_old", "lambda_new"))) |>
group_by(name) |>
mean_hdi(value, .width = .89) |>
mutate_if(is.double, round, digits = 2)# A tibble: 2 × 7
name value .lower .upper .width .point .interval
<fct> <dbl> <dbl> <dbl> <dbl> <chr> <chr>
1 lambda_old 1 0.73 1.28 0.89 mean hdi
2 lambda_new 0.43 0.24 0.61 0.89 mean hdi Because we don’t know what seed McElreath used to simulate his data, our simulated data differed a little from his and, as a consequence, our results differ a little, too.
11.3 Multinomial and categorical models
11.3.1 Predictors matched to outcomes
I will flesh this section out later.
11.3.2 Predictors matched to observations
I will flesh this section out later.
11.3.2.1 Multinomial in disguise as Poisson
Here we fit a multinomial likelihood by refactoring it to a series of Poisson models. Let’s retrieve the Berkeley data.
data(UCBadmit, package = "rethinking")
d <- UCBadmit
rm(UCBadmit)Set up the stan_data.
stan_data <- d |>
# `reject` is a reserved word in Stan, and must not be used as variable name
mutate(rej = reject) |>
select(admit, rej, applications) |>
compose_data()
# What?
str(stan_data)List of 4
$ admit : int [1:12(1d)] 512 89 353 17 120 202 138 131 53 94 ...
$ rej : int [1:12(1d)] 313 19 207 8 205 391 279 244 138 299 ...
$ applications: int [1:12(1d)] 825 108 560 25 325 593 417 375 191 393 ...
$ n : int 12Define the model_code objects for the two complimentary models.
# Binomial model of overall admission probability
model_code_11.binom <- '
data {
int<lower=1> n;
array[n] int<lower=1> applications;
array[n] int<lower=0> admit;
}
parameters {
real a;
}
model {
admit ~ binomial(applications, inv_logit(a));
a ~ normal(0, 1.5);
}
'
# Poisson model of overall admission rate and rejection rate
model_code_11.pois <- '
data {
int<lower=1> n;
array[n] int<lower=1> applications;
array[n] int<lower=0> admit;
array[n] int<lower=0> rej;
}
parameters {
real a1;
real a2;
}
model {
admit ~ poisson(exp(a1));
rej ~ poisson(exp(a2));
[a1, a2] ~ normal(0, 1.5);
}
'Compile and extract the posterior draws for both with stan().
m11.binom <- stan(
model_code = model_code_11.binom,
data = stan_data,
chains = 3, cores = 3, seed = 11)
m11.pois <- stan(
model_code = model_code_11.pois,
data = stan_data,
chains = 3, cores = 3, seed = 11)Compare the two model summaries.
print(m11.binom, pars = "a", probs = c(0.055, 0.945))Inference for Stan model: anon_model.
3 chains, each with iter=2000; warmup=1000; thin=1;
post-warmup draws per chain=1000, total post-warmup draws=3000.
mean se_mean sd 5.5% 94.5% n_eff Rhat
a -0.46 0 0.03 -0.51 -0.41 1078 1
Samples were drawn using NUTS(diag_e) at Thu Aug 15 11:47:57 2024.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).print(m11.pois, pars = c("a1", "a2"), probs = c(0.055, 0.945))Inference for Stan model: anon_model.
3 chains, each with iter=2000; warmup=1000; thin=1;
post-warmup draws per chain=1000, total post-warmup draws=3000.
mean se_mean sd 5.5% 94.5% n_eff Rhat
a1 4.98 0 0.02 4.94 5.02 2591 1
a2 5.44 0 0.02 5.41 5.47 3015 1
Samples were drawn using NUTS(diag_e) at Thu Aug 15 11:48:24 2024.
For each parameter, n_eff is a crude measure of effective sample size,
and Rhat is the potential scale reduction factor on split chains (at
convergence, Rhat=1).Though the model summaries look very different for the two models, they give the same answer, within MCMC simulation error, for the focal estimand \(p_\text{admit}\). We might explore that in a plot.
bind_rows(
# Binomial
as_draws_df(m11.binom) |>
transmute(p_admit = plogis(a)),
# Poisson
as_draws_df(m11.pois) |>
transmute(p_admit = exp(a1) / (exp(a1) + exp(a2)))
) |>
mutate(fit = rep(c("m11.binom", "m11.pois"), each = n() / 2)) |>
ggplot(aes(x = p_admit, y = fit)) +
stat_halfeye(.width = 0.89) +
scale_y_discrete(NULL, expand = expansion(mult = 0.1)) +
xlab(expression(italic(p)[admit]))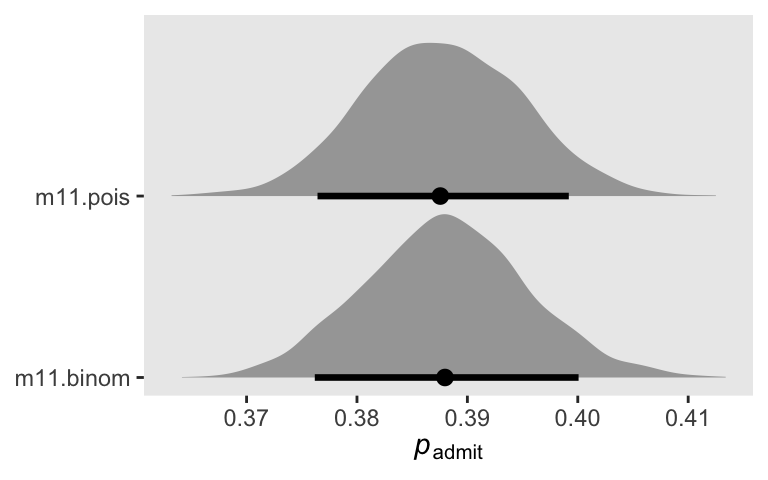
11.3.2.2 Overthinking: Multinomial-Poisson transformation
11.4 Summary
Session info
sessionInfo()R version 4.5.1 (2025-06-13)
Platform: aarch64-apple-darwin20
Running under: macOS Ventura 13.4
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/4.5-arm64/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/4.5-arm64/Resources/lib/libRlapack.dylib; LAPACK version 3.12.1
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
time zone: America/Chicago
tzcode source: internal
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] posterior_1.6.1.9000 patchwork_1.3.2 loo_2.9.0.9000
[4] rstan_2.36.0.9000 StanHeaders_2.36.0.9000 tidybayes_3.0.7
[7] lubridate_1.9.4 forcats_1.0.1 stringr_1.6.0
[10] dplyr_1.1.4 purrr_1.2.1 readr_2.1.5
[13] tidyr_1.3.2 tibble_3.3.1 ggplot2_4.0.1
[16] tidyverse_2.0.0
loaded via a namespace (and not attached):
[1] gtable_0.3.6 tensorA_0.36.2.1 xfun_0.55
[4] QuickJSR_1.8.1 htmlwidgets_1.6.4 inline_0.3.21
[7] lattice_0.22-7 tzdb_0.5.0 vctrs_0.7.0
[10] tools_4.5.1 generics_0.1.4 stats4_4.5.1
[13] curl_7.0.0 parallel_4.5.1 pkgconfig_2.0.3
[16] KernSmooth_2.23-26 Matrix_1.7-3 checkmate_2.3.3
[19] RColorBrewer_1.1-3 S7_0.2.1 distributional_0.5.0
[22] RcppParallel_5.1.11-1 lifecycle_1.0.5 compiler_4.5.1
[25] farver_2.1.2 codetools_0.2-20 htmltools_0.5.9
[28] yaml_2.3.12 pillar_1.11.1 arrayhelpers_1.1-0
[31] abind_1.4-8 tidyselect_1.2.1 digest_0.6.39
[34] svUnit_1.0.8 stringi_1.8.7 labeling_0.4.3
[37] fastmap_1.2.0 grid_4.5.1 cli_3.6.5
[40] magrittr_2.0.4 utf8_1.2.6 pkgbuild_1.4.8
[43] withr_3.0.2 scales_1.4.0 backports_1.5.0
[46] timechange_0.3.0 rmarkdown_2.30 matrixStats_1.5.0
[49] gridExtra_2.3 hms_1.1.4 coda_0.19-4.1
[52] evaluate_1.0.5 knitr_1.51 V8_8.0.1
[55] ggdist_3.3.3 viridisLite_0.4.2 rlang_1.1.7
[58] Rcpp_1.1.0 glue_1.8.0 rstudioapi_0.17.1
[61] jsonlite_2.0.0 R6_2.6.1 

Comments